Platform Problem: Why D2D'S PETITION Against modRNA/mRNA Targets Mass Deployment - Not Safe, Effective Therapeutics
Duty Calls: When medical conscience demands immediate action - Issue #18 | February 8, 2026
By W. Campbell Douglass III, MD, MS
Background
On February 6, 2026, Duty to Dissent published the public petition and related D2D post calling for a moratorium on mass-deployed modRNA products. A reader raised an important challenge: what about individualized mRNA therapies that are saving lives in rare genetic diseases? The question is fair, and it prompted the sharpening of the petition’s language — from “all current and future mRNA/modRNA applications” to “mass-deployed products.”
This post documents the technical basis for that distinction. It is intended as a reference for petition signers and anyone trying to understand why the language matters.
Link to the Petition here.
KJ’s Case Changed the Language
In May 2025, the New England Journal of Medicine published what researchers called a “milestone in the evolution of personalized therapies”: a newborn boy known as KJ, diagnosed with CPS1 deficiency — a rare and typically lethal urea cycle disorder — was treated with a custom-built, mRNA-delivered CRISPR gene-editing therapy abbreviated k-abe.
The therapy was developed by teams at the University of Pennsylvania and Children’s Hospital of Philadelphia, and manufactured in six months under an Emergency Investigational New Drug application.
The family provided genuine informed consent. The data were published transparently. There were no liability shields.
In other words, KJ’s case already meets every ethics-based standard our petition demands.
We amended the petition language because the original wording could have been read to oppose therapies like KJ’s. That was never the intent, and precision matters in our duty to dissent.
As of January 2026, KJ is 17 months old and thriving — his own cells producing his own bio-identical enzyme, playing catch, eating a normal diet, and chasing his siblings around the house (Philadelphia Inquirer, Jan. 28, 2026). This is what good medical science looks like when it functions as it should.
What KJ Received Versus What Billions Received
The COVID-19 “vaccines” manufactured by Pfizer-BioNTech and Moderna use a synthetic (modified) molecule called N1-methylpseudouridine (m1Ψ) modified RNA — commonly referred to as modRNA. In these products, every uridine nucleotide is replaced with a synthetic substitute that evades immune detection and roughly ninefold increases protein production.
KJ’s therapy was different. His treatment delivered mRNA encoding a CRISPR adenine base editor — a gene-editing enzyme.
(If your eyes just glazed over, that’s normal. Just know: it’s a tiny, precise molecular tool designed to fix one specific rare typo in one child’s DNA.)
The mRNA’s job was to enter liver cells, produce the editing enzyme, make a single targeted correction to KJ’s DNA — restoring the wild-type enzyme his body was always supposed to make — and then degrade.
The COVID modRNA products, by contrast, are the product itself — synthetic instructions designed to force cells to mass-produce a foreign protein (a modified version of the SARS-CoV-2 spike) at an industrial scale, in billions of healthy people, repeatedly — and, as Hulscher, McCullough et al. recently documented, potentially for more than 3.5 years after the last dose.
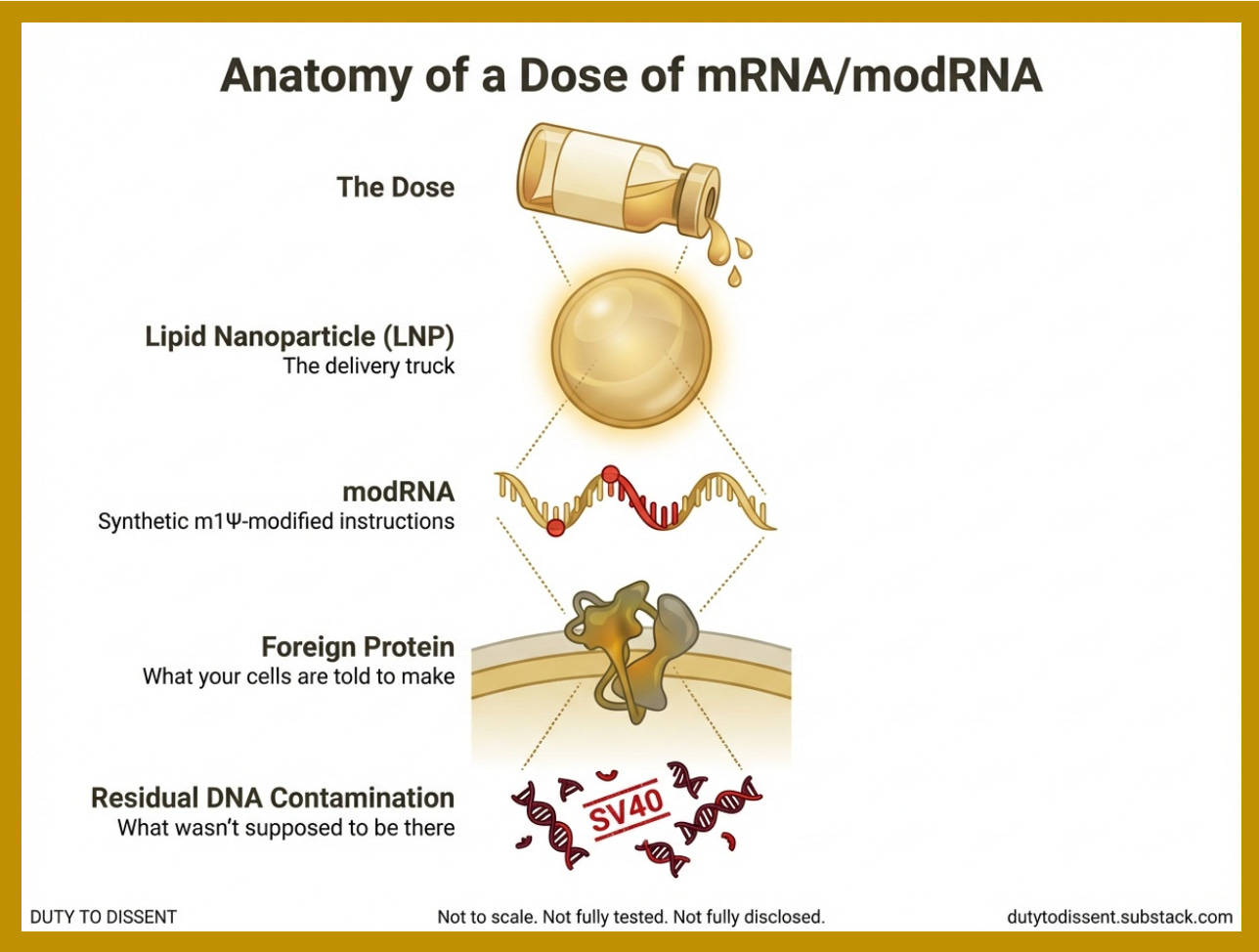
Both, however, share a common delivery platform: lipid nanoparticles (LNPs).
Shared Infrastructure: Lipid Nanoparticles
If you’re still with me after that last section, congratulations. Here’s your reward: a list of things that all require lipid nanoparticles. (If you only remember one thing from this post, make it “LNPs.” They’re the delivery truck — except instead of leaving the package at the door, it puts it directly in your bloodstream and makes stops at every organ on the route.)
This LNP shared infrastructure applies to:
COVID-19 modRNA “vaccines” (Pfizer-BioNTech and Moderna)
The LNP platform is inherently dangerous because it is inherently efficient at delivering its payloads into all cells, which has direct implications for the DNA contamination problem discussed below.
Why Mass Deployment Requires modRNA
This is not a theoretical claim. It was tested and resolved definitively.
CureVac, one of the earliest mRNA vaccine companies, built its entire platform on unmodified mRNA — no m1Ψ substitution. Their COVID-19 “vaccine” achieved only 47% efficacy, compared to over 90% for the modified Pfizer and Moderna products. In COVID-era parlance, this is called the evolution of “The Science™.” In genuine science, it’s called “being wrong.”
CureVac resisted the conclusion, ran more trials, and eventually adopted modified nucleosides for all future development. As science journalist Derek Lowe wrote in Science: “For vaccines, the question appears to have been settled.”
The implication appears to be straightforward: any mRNA product intended for mass deployment with commercially viable efficacy requires the m1Ψ nucleoside modification—modRNA.
Even “Therapeutic” mRNA Products Use modRNA
Here is a surprising detail.
Moderna’s rare disease therapeutics — the ‘good’ mRNA products — also use modRNA. From the methods section:
“Briefly, complete N1-methylpseudouridine substituted mRNA encoding hPCCA and hPCCB proteins (mRNA-3927), hMUT protein (MMA-3705), or hPAH protein (mRNA-3210) was synthesized in vitro by T7 RNA polymerase–mediated transcription from a linearized DNA template...” — Nature Communications, 2024
(Translation for those of us who didn’t memorize our biochem textbook: Moderna’s “good” mRNA products are built on the same assembly line, with the same synthetic parts, using the same delivery truck as their COVID-19 vaccine. The only difference is who gets on the truck and how many stops it makes.)
The distinction between Moderna’s therapeutic and “vaccine” products isn’t the molecular technology — it’s the scale, the consent framework, and the regulatory pathway.
DNA Contamination: A Platform-Wide Manufacturing Problem
If you thought the delivery truck problem was bad, wait until you find out what else is riding in the back. Residual DNA contamination is not unique to COVID-19 “vaccines.” It is an inherent risk of the manufacturing process used to produce all in vitro transcribed mRNA and modRNA products.
The manufacturing process: All commercially produced mRNA/modRNA is transcribed from a DNA template that “must” — ideally — be degraded and removed afterward.
As one peer-reviewed methodological analysis explains:
“Among genetically engineered drugs, those with mRNA active ingredients are a special case, as their cell-free biosynthesis requires high concentrations of DNA templates, which MUST BE REMOVED before the products can be used as drugs.”
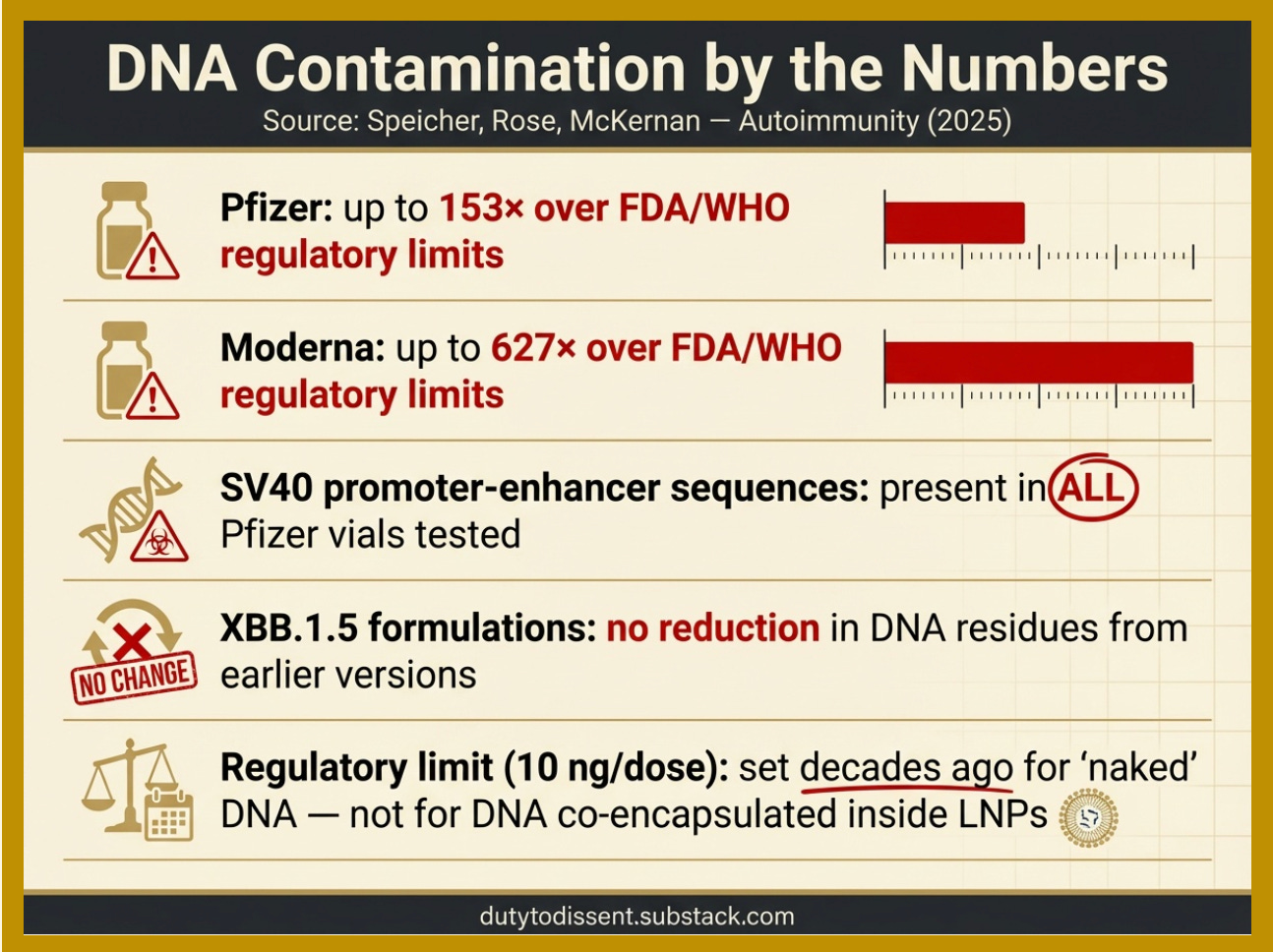
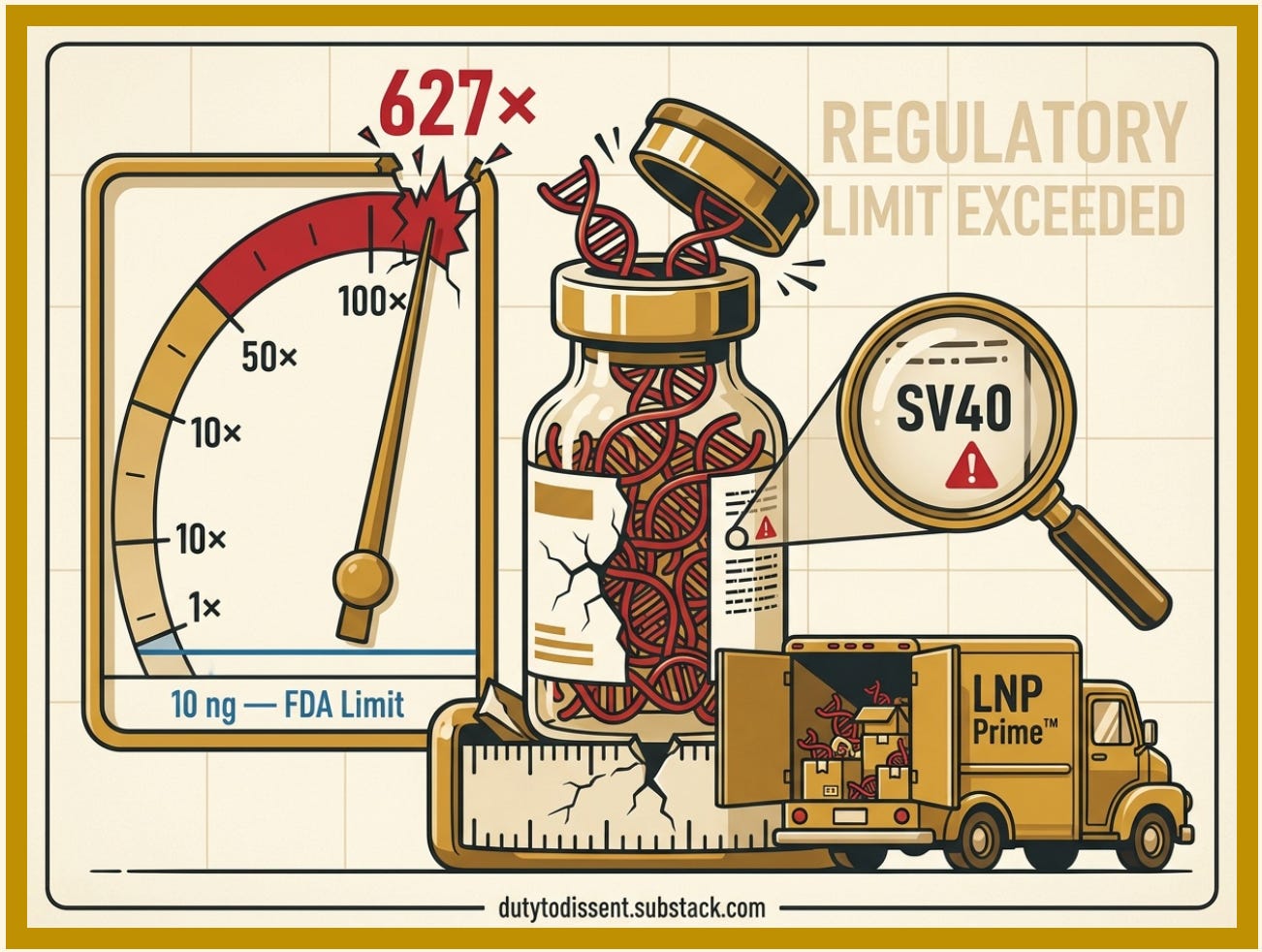
The operative word is “must.” The operative finding is that they aren’t removed by up to 627 times the regulatory limit.
The DNA Contamination of mRNA Products is Striking:
References: Speicher, Rose, and McKernan | SV40 promoter-enhancer sequences.
The amplifying factor: Existing regulatory limits for residual DNA (10 ng per dose, set by FDA and WHO) were established decades ago for ‘naked’ DNA — DNA floating freely in solution. They were not designed for DNA to be ridden inside the delivery truck. If residual DNA is co-encapsulated inside the LNPs — as researchers have reported — the effective exposure may be substantially greater than regulators assumed.
The FDA’s position: In a 2024 response letter, the FDA stated that “no safety concerns related to residual DNA have been identified.” The agency did not address the LNP co-encapsulation concern. But don’t worry — these are the same experts who said the COVID modRNA shots “stay in the arm.”
This is an unresolved scientific question with direct relevance to public safety — and it applies to the entire mRNA/modRNA manufacturing platform, not just the COVID-19 “vaccines.”
How Many People Have Received Individualized mRNA Therapeutics?
Answer: Not many.
To put the scale in perspective:
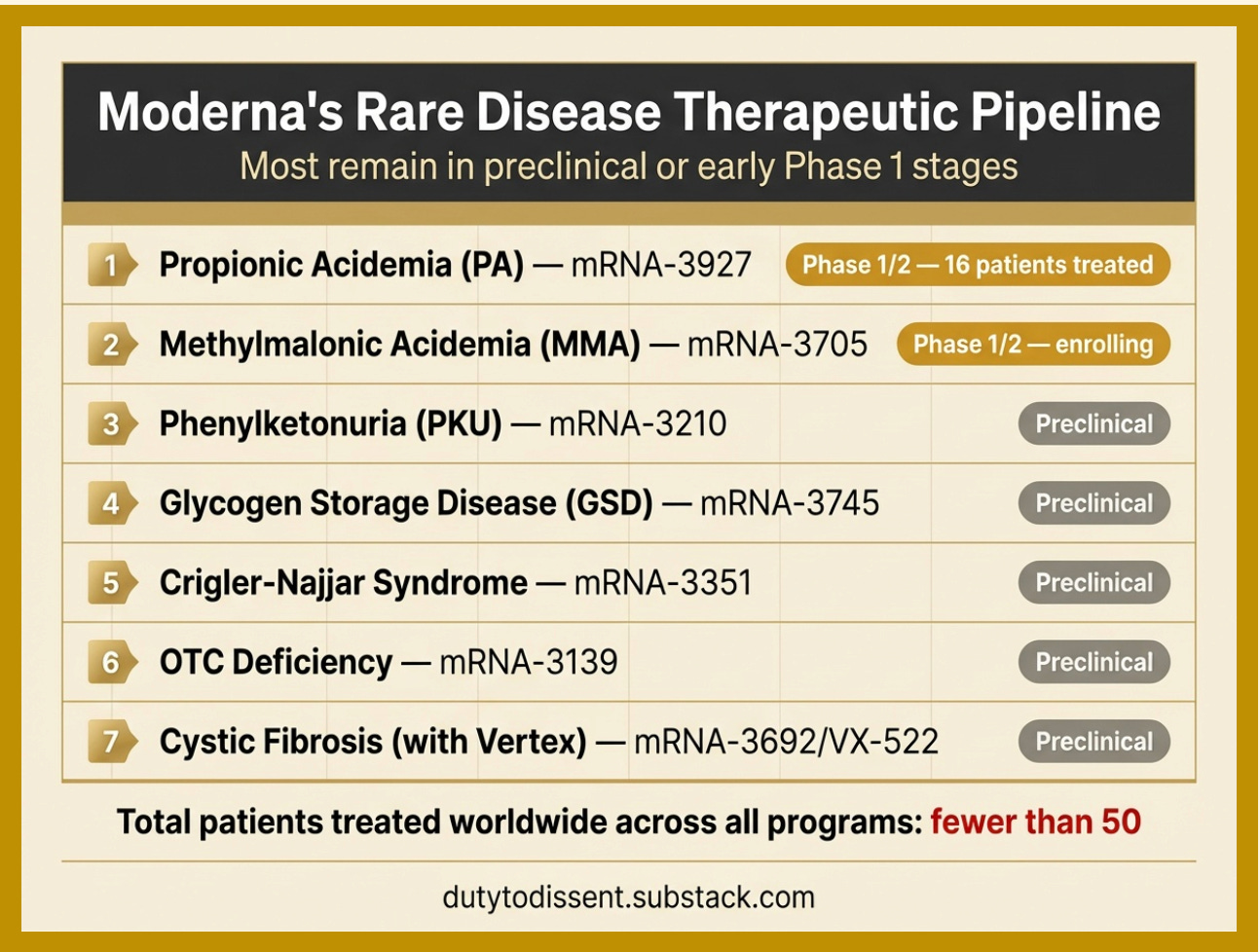
Moderna’s propionic acidemia trial (mRNA-3927) — the most advanced LNP-mRNA therapeutic program for rare genetic disease — has treated just 16 patients, with 346 total doses administered as of the most recent published data (Nature, April 2024).
KJ Muldoon is described in the literature as the world’s first recipient of a personalized in vivo CRISPR base-editing therapy.
A conservative estimate: fewer than 50 human beings worldwide have received LNP-delivered mRNA or modRNA therapeutics for the rare genetic conditions.
Compare: Billions received mass-deployed modRNA COVID-19 products. One of these numbers has a lobby. The other has a GoFundMe.”
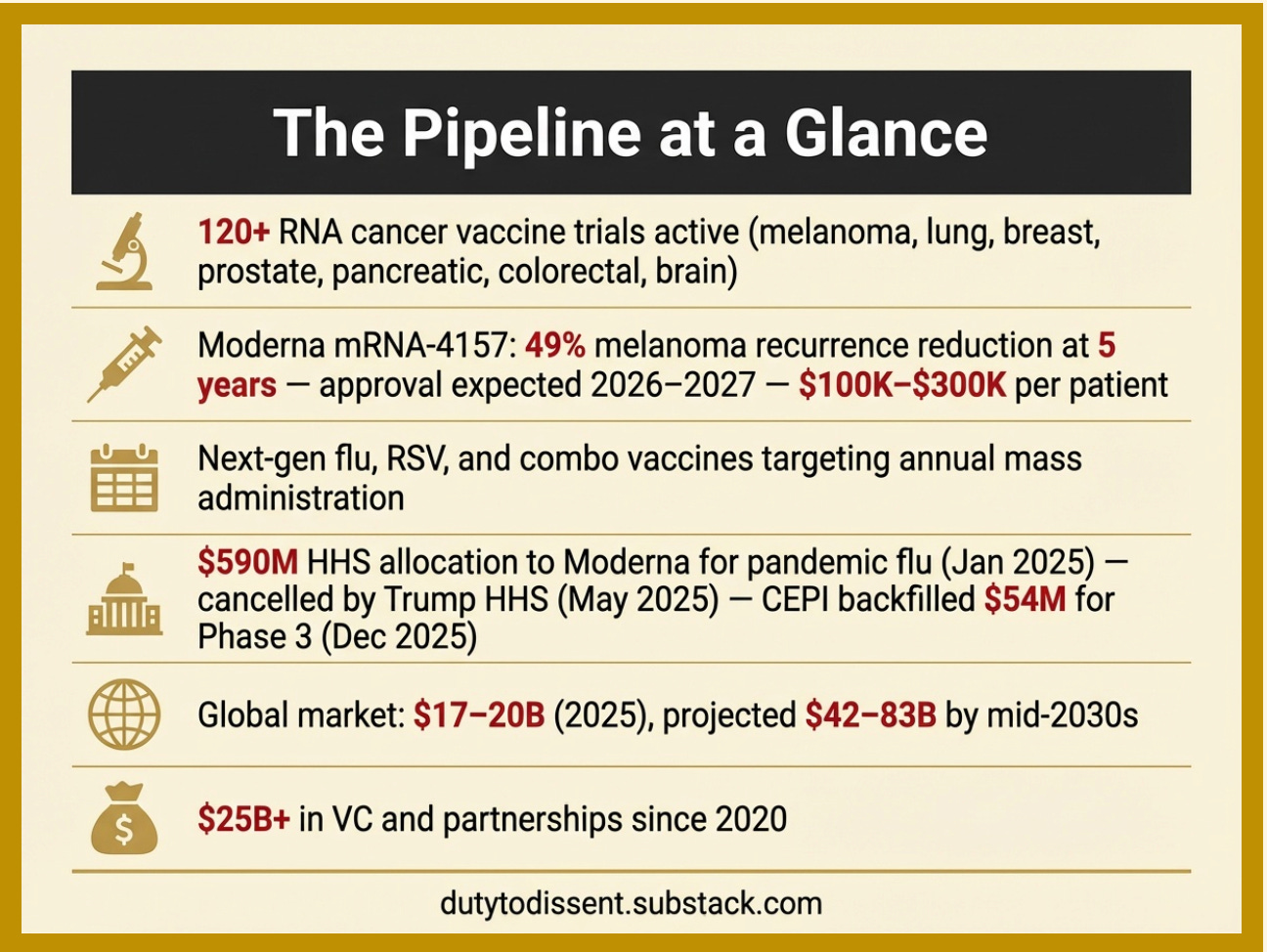
Pipeline: What’s Coming
The mRNA/modRNA platform is not contracting. It is scaling aggressively toward mass deployment.
Sources by line in the graphic, above: [1] [2] [3] [4] [5] [6]
The current market is almost entirely driven by revenues from the COVID-19 “vaccines,” which are declining dramatically. Turns out “safe and effective” loses its marketing magic when 4,000+ studies say otherwise. The massive growth projections depend on pipeline products — cancer vaccines, flu vaccines, and pandemic platforms — that have not yet been approved. This creates a significant financial incentive to push mass-deployment products through regulatory approval regardless of unresolved safety questions.
Micro-Needle Patch Implants
And the pipeline doesn’t end at vaccines and therapeutics. In October 2025, D2D documented Gates-funded research—published in Nature and developed at MIT and Harvard— that combines modRNA delivery with quantum-dot biometric tracking via microneedle patch implants. Same LNP platform. New cargo. Gates himself, on video: ‘We just need to mess around with the lipid nanoparticles.’ (Read: Microneedle Patch Implant — Will Your Silence Ensure It Succeeds?)
If you’ve made it this far, you now know more about modRNA platform technology than most members of Congress who are voting to fund it.
What the Petition Targets — and What It Doesn’t
Our petition calls for a moratorium on mass-deployed modRNA products pending independent safety review, full transparency, informed consent, and the removal of manufacturer liability protections.
Does not target: Individualized mRNA therapeutics for rare diseases with informed consent (like KJ’s case), or basic research conducted transparently.
Does target: Population-scale modRNA deployment under coerced consent, suppressed safety data, total manufacturer liability immunity, and platform expansion into cancer, flu, and pandemic products without addressing unresolved safety concerns.
Three states have now introduced legislation classifying mRNA injections as biological weapons of mass destruction. Our petition takes a different path — a moratorium pending review, but the direction is the same: accountability.
The distinction reflects a principled difference between medicine practiced with transparency, consent, and accountability — and an industrial platform built for mass administration under conditions that lack all three. These therapeutic applications already meet every standard this petition demands for mass-deployed products. That contrast is the indictment.
This post is a reference document supporting the Duty to Dissent public petition. The petition is currently at 1,751 signatures in less than 48 hours. If you have not yet signed, you can do so here.
If you believe we have made an error of fact in this post, contact us. We correct mistakes. That is what distinguishes dissent from dogma.
Sources and Key References
Musunuru K, et al. “Patient-Specific In Vivo Gene Editing to Treat a Rare Genetic Disease.” N Engl J Med. 2025 May 15. DOI: 10.1056/NEJMoa2504747. Full text
Aldevron/IDT. “Manufacture World’s First mRNA-based Personalized CRISPR Therapy.” May 15, 2025. Press release
Gropman A, Komor A. Editorial accompanying Musunuru et al. NEJM. 2025. Via Springer Medicine summary
Nair N, et al. “Characterizing the mechanism of action for mRNA therapeutics for the treatment of propionic acidemia, methylmalonic acidemia, and phenylketonuria.” Nat Commun. 2024. PMC full text — Contains methods confirming N1-methylpseudouridine substitution in Moderna therapeutics and linearized DNA template manufacturing
An D, et al. “Interim analyses of a first-in-human phase 1/2 mRNA trial for propionic acidaemia.” Nature. 2024 April 22. Full text
Nance KD, Meier JL. “Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines.” ACS Cent Sci. 2021. Full text
Cohen J. “What went wrong with CureVac’s highly anticipated new mRNA vaccine for COVID-19?” Science. 2021. Full text
Lowe D. “CureVac Comes Around.” Science (In the Pipeline blog). Full text
Bernard MC, et al. “The impact of nucleoside base modification in mRNA vaccine is influenced by the chemistry of its lipid nanoparticle delivery system.” Mol Ther Nucleic Acids. 2023. PMC full text
Uddin MN, Roni MA. “Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines.” Vaccines. 2021. PMC full text
Speicher DJ, Rose J, McKernan K. “Quantification of residual plasmid DNA and SV40 promoter-enhancer sequences in Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada.” Autoimmunity. 2025. Full text | PubMed
König J, Kirchner T. “Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®.” Methods and Protocols. 2024. PMC full text
FDA. Response letter regarding SV40 promoter/enhancer DNA in mRNA COVID-19 vaccines. 2024. PDF
Chaudhary N, et al. “Lipid-Nanoparticle-Based Delivery of CRISPR/Cas9 Genome-Editing Components.” Mol Pharm. 2022. Full text
Current Progress and Future Perspectives of RNA-Based Cancer Vaccines: A 2025 Update. Vaccines. 2025. PMC full text
“Merck-Moderna cancer vaccine sustains 49% melanoma risk reduction at 5 years.” Fierce Biotech. January 2026. Full text
“Cancer Vaccines 2025: The Rise of mRNA Therapies.” Cromos Pharma. November 2025. Full text
Liu C, et al. “mRNA therapies: Pioneering a new era in rare genetic disease treatment.” J Control Release. 2024. Abstract
Moderna. Rare Disease Clinical Trials. Portal
“Personalized Base Editing Treats Infant With Rare Urea Cycle Disorder.” Precision Medicine Online. May 2025. Full text